Continuous Purification: CaptureSMB
Continuous CaptureSMB processes—biologics
During the production of monoclonal antibodies, the Protein A capture step is the first important purification step. A fast capture step is critical to retaining product integrity and processing of large feed volumes in reasonable times. Novel periodic counter-current (PCC) processes are superior to batch processes, offering a significant economic benefit.
YMC’s optimized two-column periodic countercurrent chromatography process (CaptureSMB) is the least complex and most effective process, allowing two to threefold faster processing. It saves up to 60% of expensive Protein A resin and buffer. Lab (Contichrom CUBE instrument) and GMP scale production systems (Contichrom TWIN LPLC) employing CaptureSMB are commercially available.
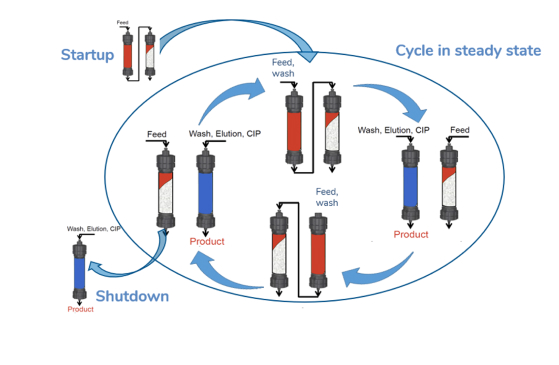
CaptureSMB resources
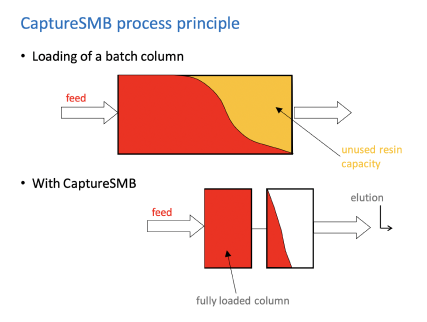
The CaptureSMB Process Principle

CaptureSMB for the continuous purification of mAbs with high productivity and load

Capture SMB; Twin-column chromatography—how it works, economics, and applications
Application Search
Use our extensive Applications Search page to mine a dynamically growing collection of over 350 YMC-produced application notes.
The data can be searched by any combination of several variables, including sample classification, compound name (including partial names), and column parameters.
