Oligonucleotides
Leading Innovation in Oligonucleotide Purification
Production of Oligonucleotides
Oligonucleotides are emerging as the platform for the future of new therapies, including new (mRNA) vaccines. The effectiveness and safety of oligos for a wide variety of transmissible and genetic diseases lends great promise for future treatments. YMC has been at the side of peptide and oligo scientists for four decades—with phases, resins, lab services, and now innovative multi-column chromatography designs.
YMC is the global leader in purification solutions for oligo CMOs and end users. For example, our metal-free columns deliver improved sensitivity and peak shape of coordinating compounds such as nucleotides or oligonucleotides. And our industry-leading Contichrom twin-column continuous purification systems, enabled with our patented MCSGP* process, enhance oligo yield up to 60% and reduce time in production up to 40% while reducing QC analysis by up to a factor of 10.
The production of oligonucleotides for therapeutic use requires that any undesired byproducts of synthesis be removed in order to obtain pharmaceutical-grade purity. To this end, a chromatographic polishing step is routinely undertaken using YMC columns and phases/resins. The conventional batch approach often has an overall yield of production of 40-60%. Given the high cost of synthesis, poor chromatographic performance downstream has a major negative impact on the economics of the overall production. As a countermeasure, lower purity “waste” products can undergo re-chromatography; however, this greatly reduces process productivity and significantly increases the burden of analytical characterization of product fractions. Thus, manufacturers are looking for alternative, more advanced chromatographic methodologies to boost yield. YMC now provides the solution to the yield vs. purity trade-off, with substantial savings in production time.

Economic example of one YMC technology
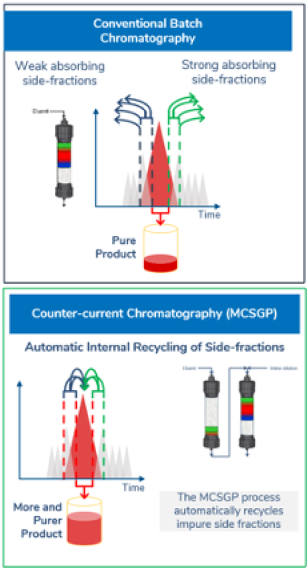
The continuous chromatography process MCSGP* leads to higher yields at target purity. MCSGP is a chromatographic method operated continuously using two columns, compared to the batch mode of single-column chromatography (see graphic). Much of the basic principle, however, remains the same. In a chromatographic process, the impure feed mixture is separated, giving fractions with pure product, fractions with a mixture of impurities and product, and fractions containing only impurities. Pure product fractions are collected and impure product-containing side fractions are discarded or re-processed.
If product containing side fractions are discarded, valuable product is lost. Re-processing the side fractions accumulates impurities and applicability is thus limited. Moreover, reprocessing reduces overall productivity. MCSGP can isolate most of the product included in the starting material by internally recycling product-containing side fractions and repeatedly separating impurities and pure product. The recycling is done automatically, without the need for offline analysis. The impure side fractions are transferred from one chromatographic column to another. Fresh feed material is added every cycle. This ensures continuous operation and leads to a product with high yield without compromising purity.
Purification of MCSGP* with Contichrom technology
MCSGP offers significant advantages over single-column chromatography in the purification of oligonucleotides. These advantages include:
- An increase in oligonucleotide yields of at least 50%, leading to over 90% recovery at 92% purity (target purity was >91%)
vs. 60% recovery for single-column chromatography - An increased yield that allows massive scale-down of oligonucleotide synthesis upstream to obtain required oligo amounts
- A twofold increase in productivity compared to the benchmark run
- A massive reduction in the number of samples for analytical characterization, especially if re-chromatography of waste fractions is carried out. (Only one sample is generated per MCSGP cycle for two feed injections, while multiple fractions per injection are generated in single-column chromatography.)
- A high yield that makes re-chromatography obsolete
The increase in productivity by MCSGP allows multiple manufacturing options:
- The same target amount can be produced within the same time with smaller columns
- A specified target amount can be produced in a shorter period of time with columns of the same size
- More target compounds can be produced per total column volume in the same amount of time

*MCSGP = Multicolumn Countercurrent Solvent Gradient Purification
Application Search
Use our extensive Applications Search page to mine a dynamically growing collection of over 350 YMC-produced application notes.
The data can be searched by any combination of several variables, including sample classification, compound name (including partial names), and column parameters.
